Timeline Of Eminent Spine
August 2024
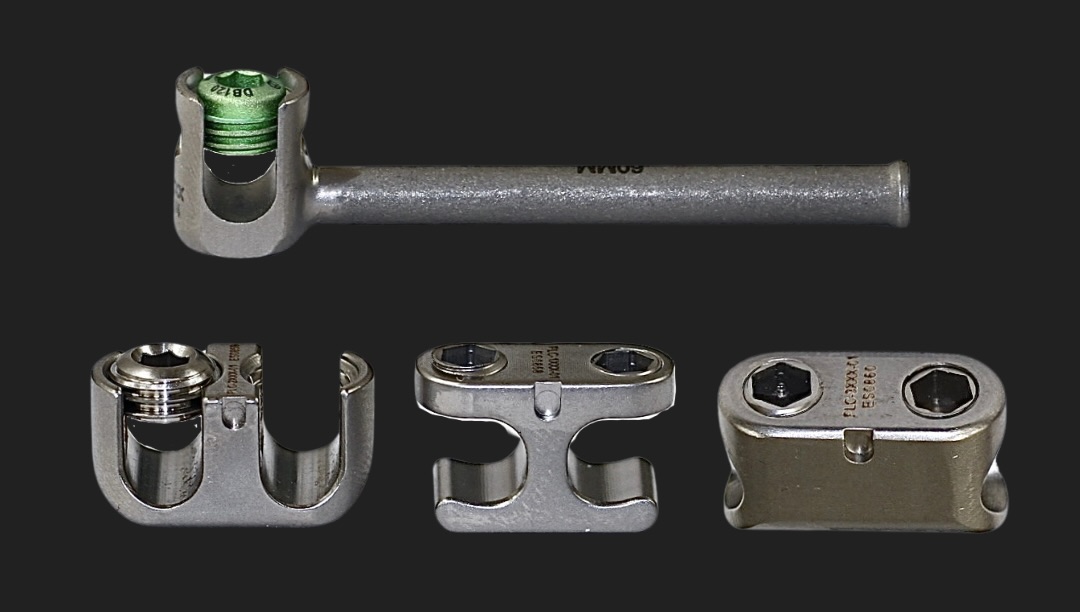
Eminent Spine’s scoliosis Deformity PEDICLE SCREW SYSTEM.
US-FDA 510(K) CLEARANCE AS OF August 20, 2024.
June 2024

Eminent Spine’s 3D Titanium and Machined Titanium SI Screw System.
US-FDA 510(k) Clearance AS OF June 4, 2024.
May 2023
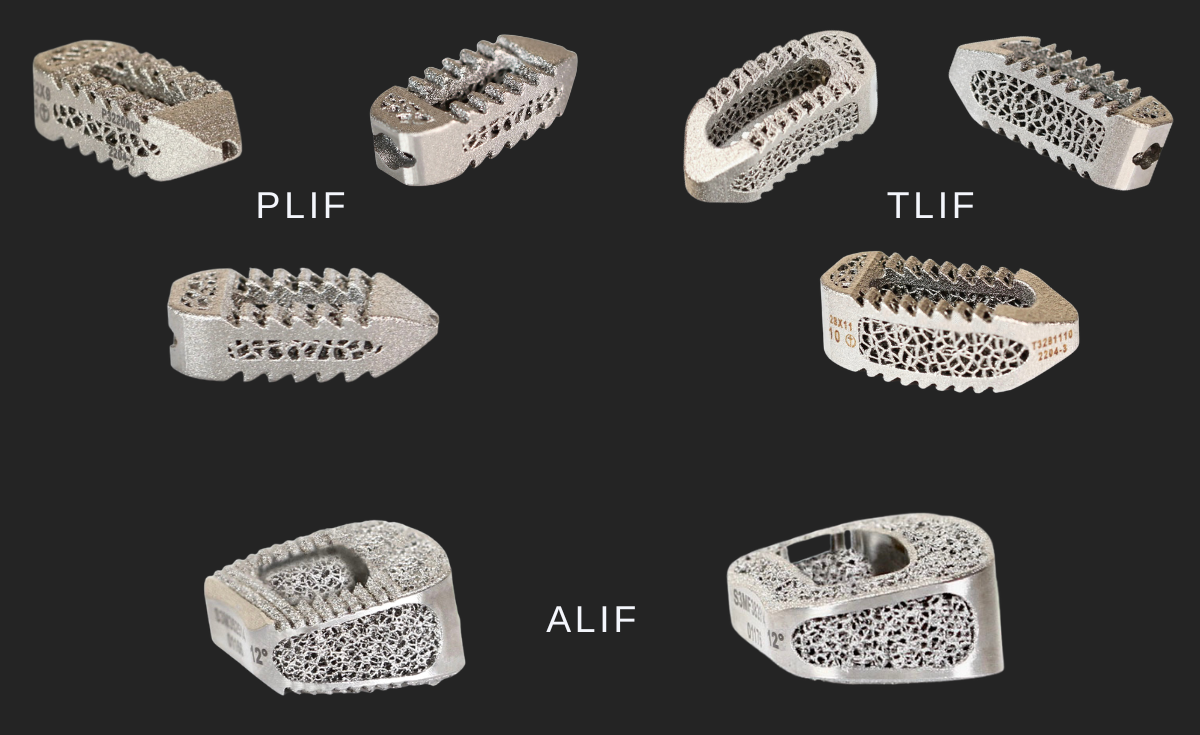
Eminent Spine’s 3D Titanium lumbar interbody fusion systems: PLIF/TLIF/ALIF.
US-FDA 510(k) Clearance AS OF may 15, 2023.
February 2023

Eminent Spine’s Cervical 3D Titanium cages are OFFERED non-sterile.
US-FDA 510(k) Clearance AS OF February 6, 2023.
October 2022

EMINENT SPINE’S LUMBAR STAND-ALONE SYSTEM INCLUDES NON-STERILE IMPLANTS WITH 5 IMPLANT PROFILES AND hyperlordotic angles.
lumbar PEEK & 3D TItanium CAGES ARE available IN OUR STAND-ALONE SYSTEM.
US-FDA 510(K) CLEARANCE AS OF october 17, 2022.
November 2021





EMINENT SPINE’S CERVICAL STAND-ALONE SYSTEM INCLUDES NON-STERILE IMPLANTS WITH A WIDE RANGE OF IMPLANT PROFILES WITH BOTH FIXED AND VARIABLE SCREW OPTIONS.
CERVICAL PEEK, TITANIUM & 3D TI CAGES ARE OFFERED IN OUR STAND-ALONE SYSTEM.
US-FDA 510(K) CLEARANCE AS OF NOVEMBER 5, 2021.
January 2021
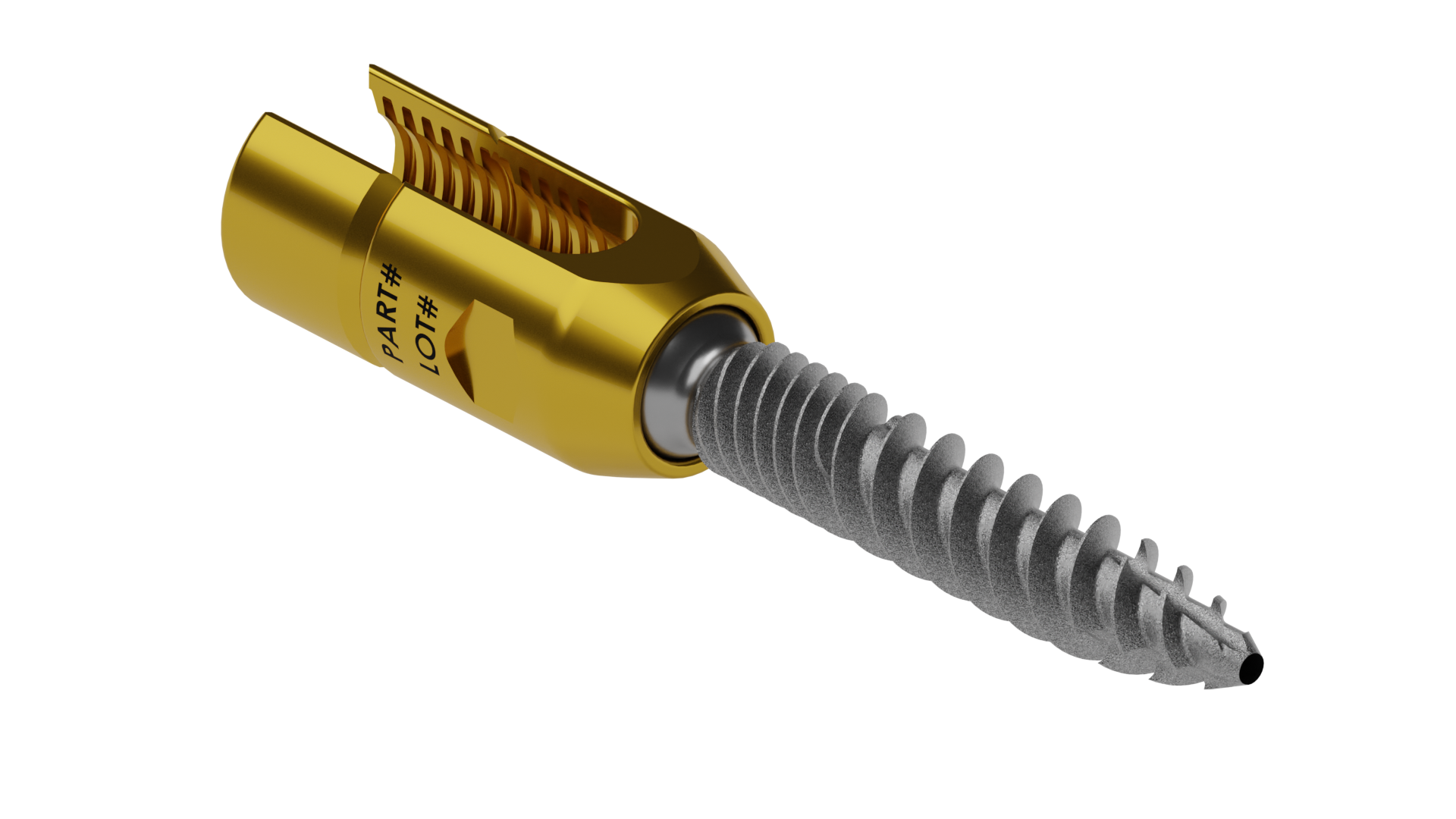
RELEASE OF SURFACE TEXTURE PEDICLE SCREW.
RELEASED 4.75MM CANNULATED PEDICLE SCREW.
November 2020

rELEASE OF C3 & s3 PEDICLE SCREW (TAPERED TIP & tHICKER NECK).
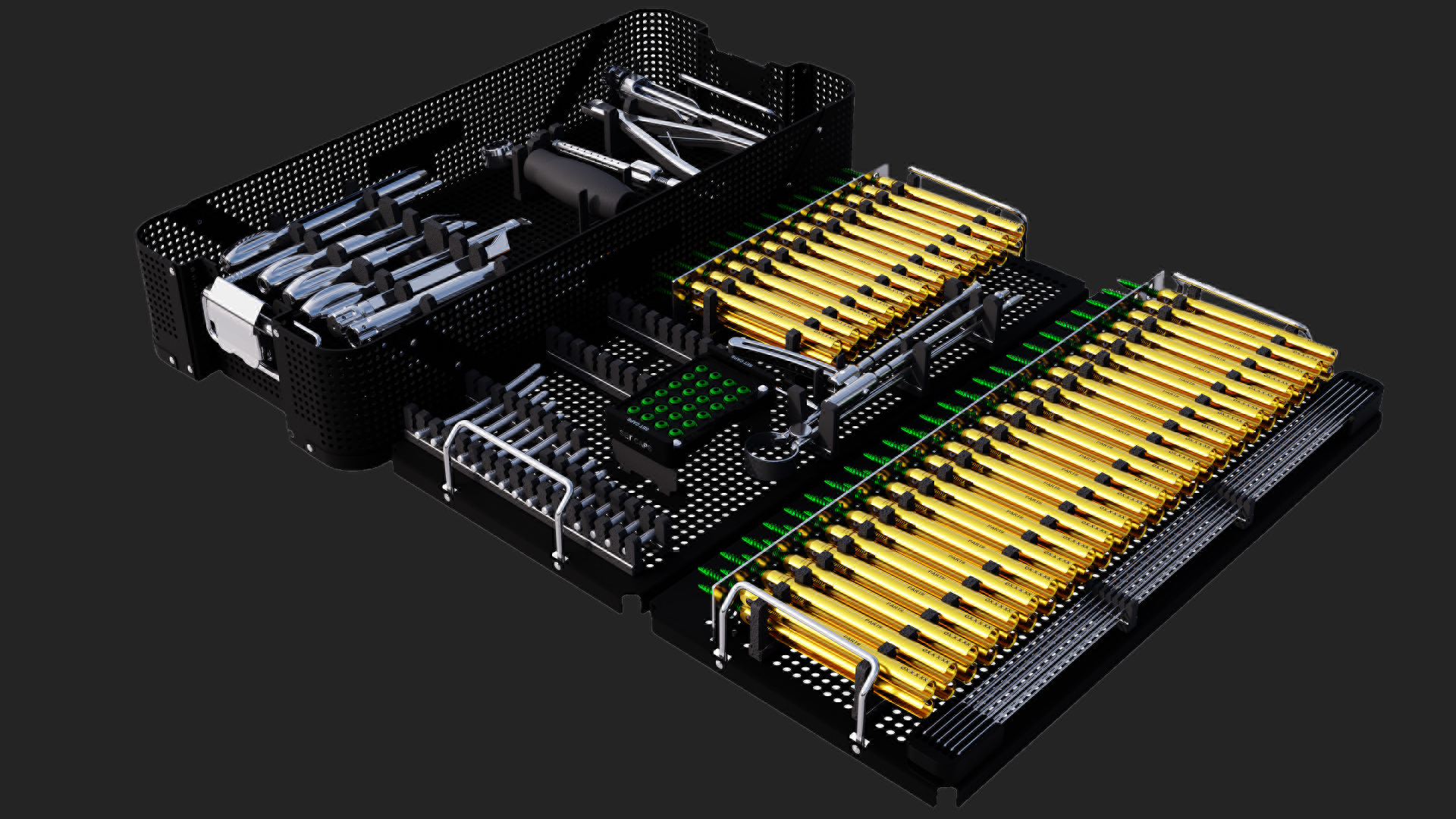
Release of 4.00MM MIS Pedicle Screw Towers & INSTRUMENTATION DELIVERY SYSTEM.
Release of Grit Blasted Pedicle Rod with improved Lordosis & Marking.
October 2020

US-FDA 510(K) CLEARANCE FOR THE MODERN, LOW-PROFILE ANTERIOR CERVical plate system.
June 2020
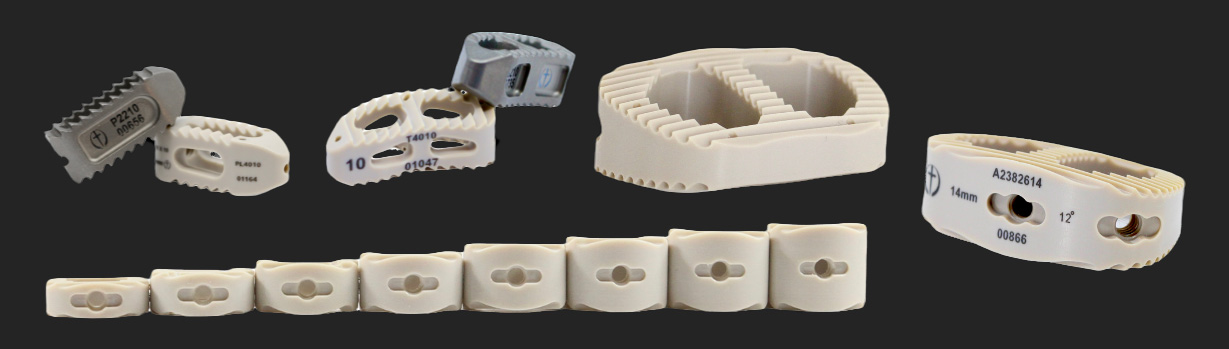
LUMBAR AND CERVICAL PEEK IBFD SYSTEM UPDATED.
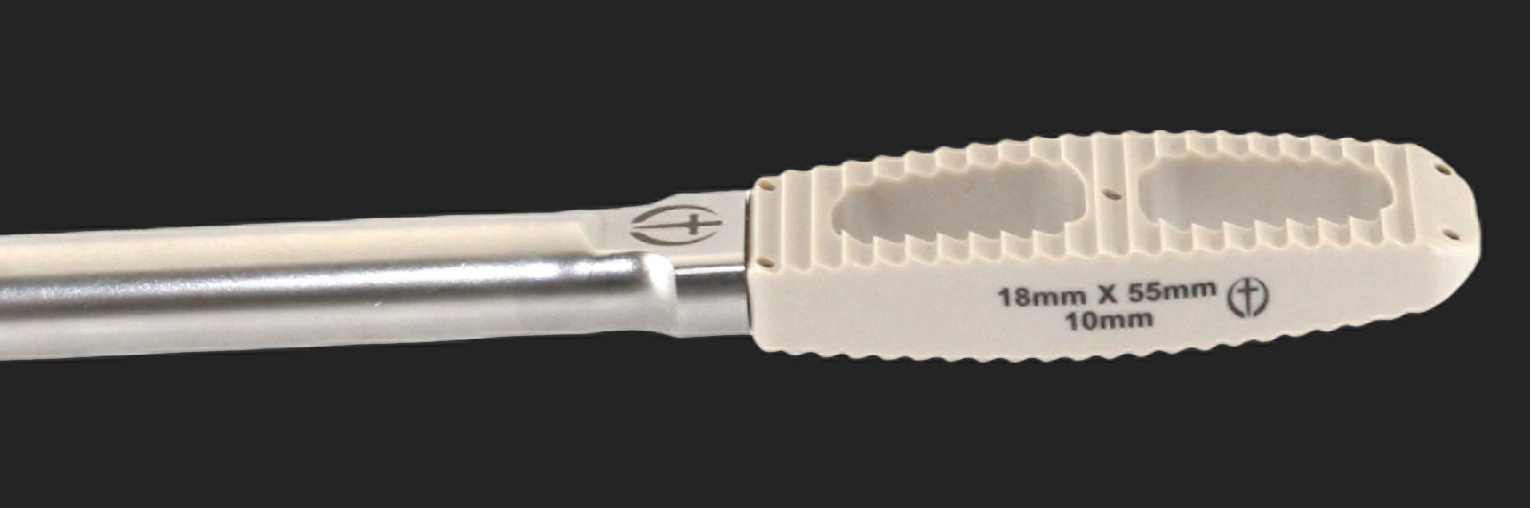
LUMBAR PEEK LATERAL IBFD SYSTEM UPDATED.
February 2020

EMINENT SPINE UNDERWENT FULL TRANSFORMATION INCLUDING REBRANDING, ADMINISTRATION CHANGES AND NEW FDA CLEARANCES.
RELOCATED TO NEW HEADQUARTERS IN PLANO, TX.
2015
US-FDA 510(K) CLEARANCE FOR EMINENT EXTREMITIES.
2014
US-FDA 510(K) CLEARANCE FOR THE ANTERIOR LUMBAR PLATING SYSTEM.
2013
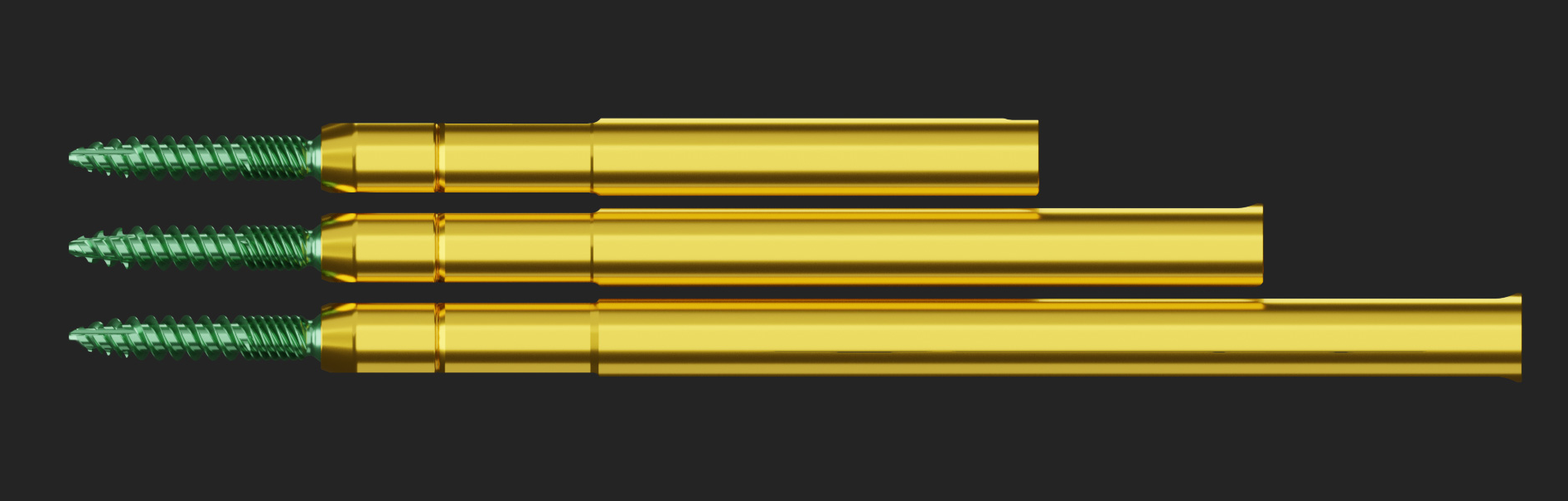
Pedicle MIS System released.

lumbar peek lateral cage system released.
2012
INTERNATIONAL SPINAL SALES BEGINS.
2011
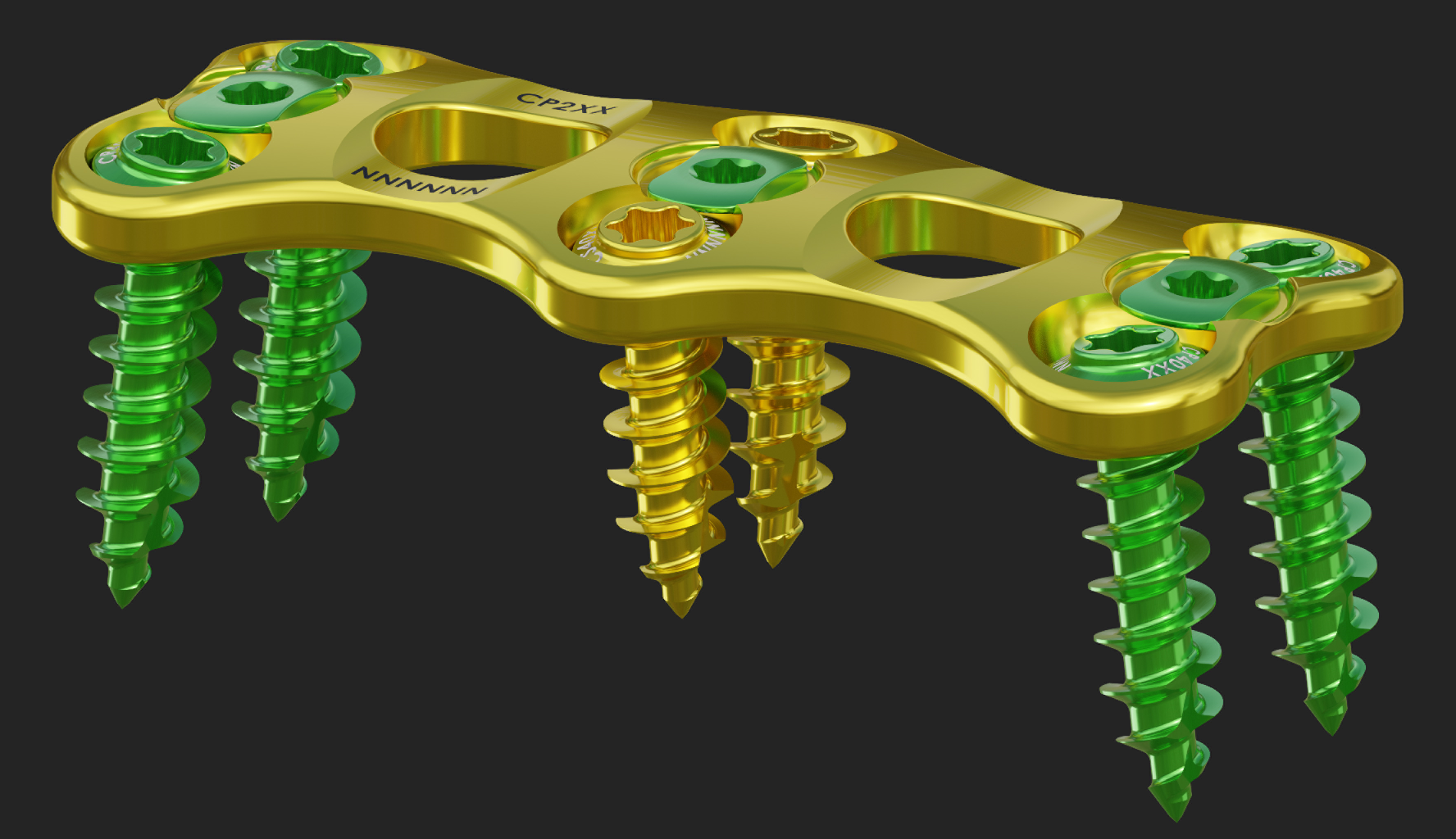
US-FDA 510(K) CLEARANCE FOR THE ANTERIOR CERVICAL PLATING SYSTEM.
EMINENT SPINE RELEASES THE ANTERIOR CERVICAL PLATE CLINICAL DATA.
EMINENT SPINE RELEASES THE LUMBAR PEDICLE SCREW & CROSS-LINK CLINICAL DATA.
2010

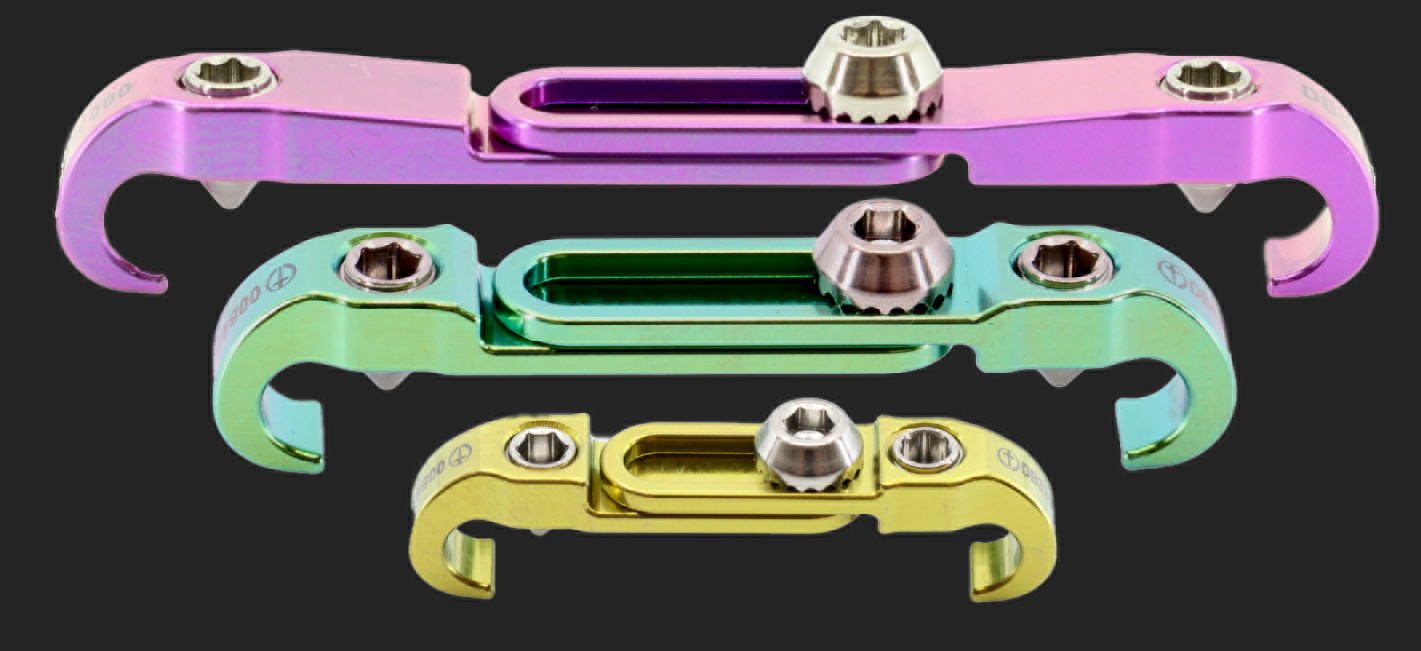
US-FDA 510(K) CLEARANCE FOR THE CANNULATED & NON-CANNULATED PEDICLE SCREW SYSTEM & THE LUMBAR CROSS-LINK.
2009

US-FDA 510(K) CLEARANCE FOR THE PEEK INTERBODY FUSION SYSTEM.


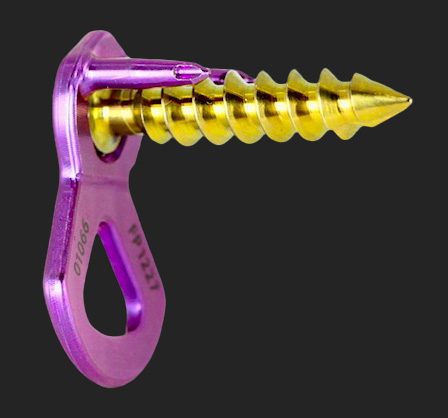
US-FDA 510(K) CLEARANCE FOR THE ANTERIOR LUMBAR BUTTRESS SYSTEM.
2008
EMINENT SPINE WAS FOUNDED.

